Medical Implants.
Quartus engineers have years of experience designing, analyzing, and testing medical devices throughout the whole body, including performing one-off simulations, assisting with submission materials and complex design-of-experiments. From single-use products, to wearable products, to permanent implants, the Quartus team has years of experience in areas such as:
- Cardiac implantable and disposable devices
- Orthopedic implants and tools
- Otolaryngology devices
- Single-use, and multi-use test equipment
- Prosthetics
CAPABILITIES
Structural, Thermal, Fluid Flow, Multiphysics
- Design, assembly, integration, and product simulation
- ASME V&V 40 (Verification & Validation in Computational Modeling of Medical Devices)
- In vitro/vivo modeling
- Fluid-structure interface modeling for full device characterization
Simulation Based Acute and Fatigue Response
- Radial force simulations and correlations
- Infinite life characterization
- Material characterization and calibration
- Metallic, ceramic, and biological materials
Nitinol Subject Matter Experts
- Nitinol device design
- Shape setting optimization
- Training and educational services available
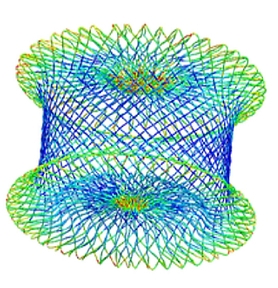
contact nitinol wire braid
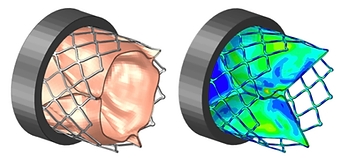
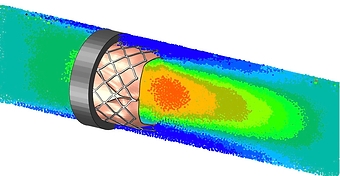
CASE STUDY
Fatigue & Hemodynamic Analysis of Aortic Heart Valve
- Fully-coupled fluid-structure analysis using StarCCM & Abaqus
- Fluid dynamically deflects leaflet tissue, imparting physiological loads on stent
- Elasto-plastic, superelastic, & custom anisotropic hyperelastic material models
- Full-field stresses & strains on stent, leaflets, & fluid
Quartus aids the design process through:
- Manufacturing & fatigue analysis of the stent frame
- Allows for rapid design iterations
- Nitinol, stainless steel, titanium; tube or braid
- Hemodynamic analysis
- Calculation of pressure drop, stagnation, hemolysis, etc.
- Trade studies on geometric & manufacturing parameters
- Regulatory submission analyses
- Verification & validation
- Qualitative & quantitative comparison to in-vitro testing
- Convergence & sensitivity studies
